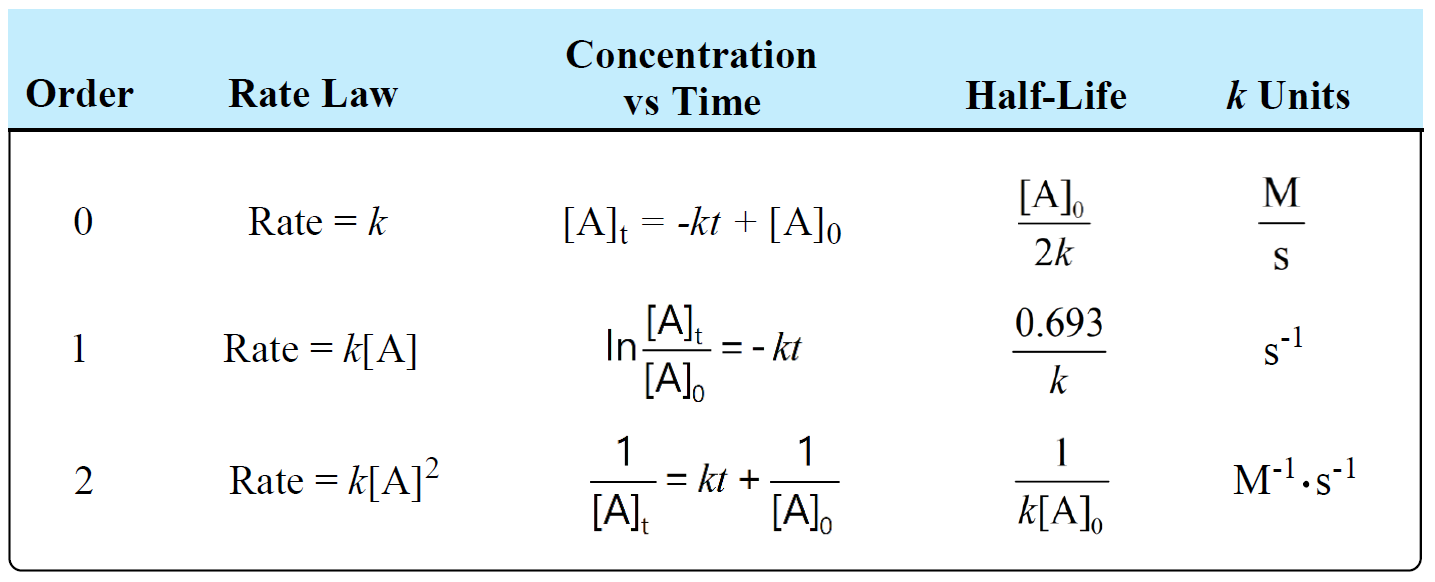
half life formula for first order reaction
Half Life period of first order reaction is the time required for 50 percent completion of the reaction and is represented as t 05 ln 2 K or Half Life Period ln 2 Rate constant. Where The half-life of a reaction is referred to as t.

Rate Equation For First Order Reactions
However for catalytic reactions at low concentrations they are no longer linear since they stop being zero-order reactions.
. Half Life Calculator first order reaction input the equations calculated rate constant. L n A B k. Added Dec 9 2011 by ebola3 in Chemistry.
Using the half-life equation derived from the concentration-time equation as shown in example 1 we can solve for the initial concentration of reactant. Graphical relations and half lives. Substituting the values in the.
For a zero order reaction A products rate k. The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02. T 12 0693k.
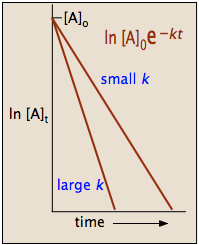
It is a constant and related to the rate constant for the reaction. What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions. T_12 frac1A_0k.
So here is your half-life for a first order reaction. So our half-life is equal to let me rewrite this here so our half-life t 12 is equal to 693 divided by k where k is our rate constant. Converting a half life to a rate constant.
Determining a half life. We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. The half-life of a first-order reaction is given as t 12 0693k.
The half-life of a second-order reaction is given by the formula 1kR 0. The half-life of a first-order reaction is given as t 12 0693k. 1 A k t 1 A 0.
The integrated rate equation for the second type of second-order reaction is. The half-life of a first-order reaction does not depend upon the concentration of the reactant. T ½ A o 2k.
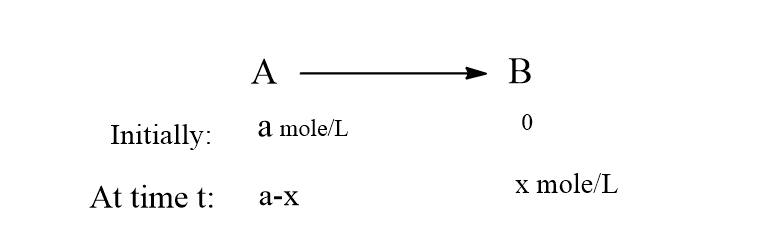
Rate Constant for First Order Reaction 2303 Time for completion Initial Reactant A Concentration-Initial Reactant B Concentrationlog10Initial Reactant B Concentration. The integrated rate equation for the first type of second-order reaction is. By rearranging the above equation the half-life of a first-order reaction can be obtained to be t_12frac2303klogleft 2 right t_12frac0693k.
The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant. This widget calculates the half life of a reactant in. The half-life of a species is the time it takes for it to.
Ln left fracA_0A_t rightkt tln. The half-life of a second-order reaction is given by the formula 1kR 0. Equations for Half Lives.

The Half Life Period Of Any First Order Reaction
Rate Equation For First Order Reactions
I What Is Meant By Half Life Period Of A Reaction Ii By Deriving The Equation For T1 2 Of First Order Reaction Sarthaks Econnect Largest Online Education Community
2 3 First Order Reactions Chemistry Libretexts

Zero Order Reaction Definition Examples Formula
First Order Reaction Definition Example Half Life Period Chemistry Notes
Units Of Rate Constant K Chemistry Steps

Half Life Of Zero Th 0th Order Reaction Derivation Youtube

Zero Order Reactions Video Kinetics Khan Academy
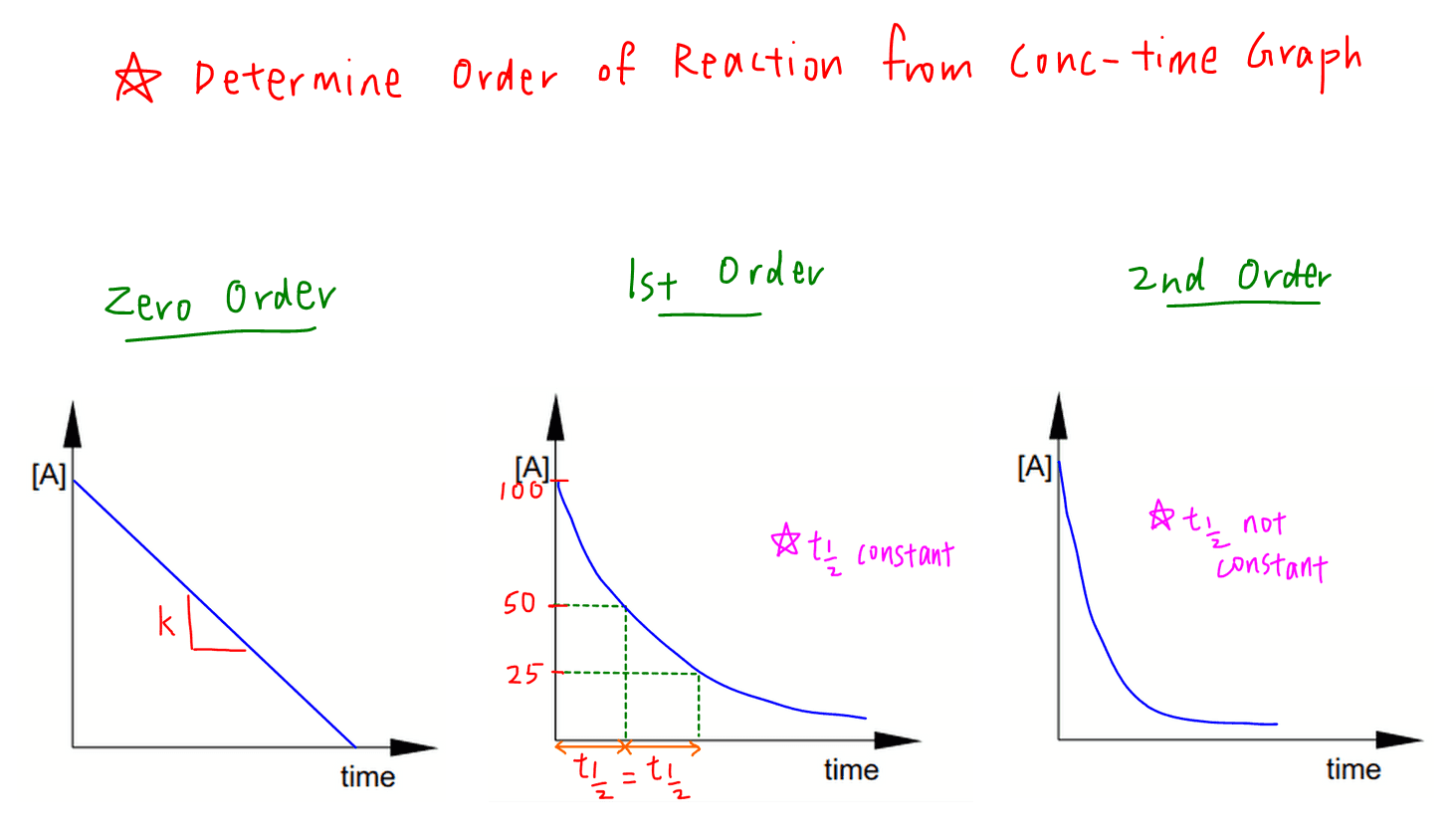
Rate Equation And Order Of Reaction

First Order Rate Constant An Overview Sciencedirect Topics

Cbse Class 12 Science Answered

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

Half Life Of A Second Order Reaction Derivation Youtube

First Order Reaction Definition Example Half Life Period Chemistry Notes